
Hamza Hbil
Radboudumc
Nijmegen, the Netherlands

Radboudumc
Nijmegen, the Netherlands

Centre de Recherches Médicales de Lamabaréné (CERMEL)
Gabon

TASK
Cape Town, South Africa

The PanACEA SUDOCU study completed its recruitment: a total of 75 participants were randomized to 5 arms (15 participants each) with a wide range of sutezolid doses (from 0mg to 800mg BID given over three months). With this open-label, randomized, phase 2B, dose selection study (ClinicalTrials.gov identifier NCT03959566) we aim to describe the safety/tolerability-exposure relationship…

Ludwig Maximilian University
Munich, Germany

Ludwig Maximilian University
Munich, Germany

An interview with prof. dr. Martin Boeree A considerably higher dose of the anti-tuberculosis drug rifampicin has been found to be safe according to a recent publication by PanACEA. Higher dosing of rifampicin may lead to a shorter / more efficacious treatment for tuberculosis with less resistance development. With this finding PanACEA researchers complete a…
Read more Shorter treatment may be possible with higher rifampicin doses
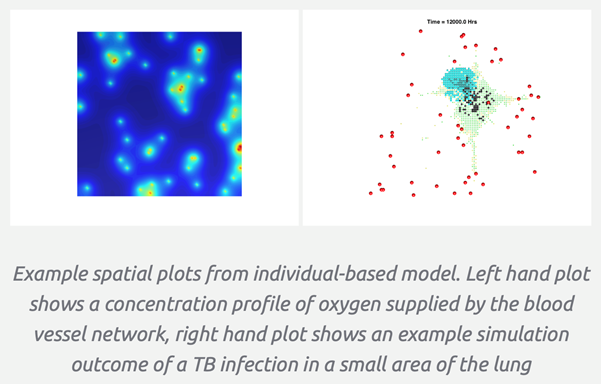
The St Andrews group’s most recent paper has been published as a perspective piece in the International Journal of Tuberculosis and Lung Disease.The collaboration between the Schools of Medicine, Applied Mathematics and Computer Science provides an overview of the different types of model that can be applied to tuberculosis clinical trials. Importantly, it provides a…

In the afternoon of 20th of February a ninety minutes interactive workshop on community engagement was held. The inspiring Erica Sanga, Community Engagement Coordinator at NIMR Mwanza, Tanzania, led the workshop. During this workshop, participants from different sites shared their experiences and challenges on community engagement activities. Participants discussed their best practices for successful engagement…

Delegates from all of our collaborators in SimpliciTB TB travelled from across the world to attend the annual face to face General Assembly (GA) meeting in Mbeya, Tanzania. Trial progress was reported, with a focus on recruitment and medical monitoring. An important development was the launch of the Capacity Development programme that allows members of…